Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ohira, Saki; Abe, Takeyasu; Iida, Yoshihisa
Radiochimica Acta, 111(7), p.525 - 531, 2023/07
Times Cited Count:0 Percentile:0.01(Chemistry, Inorganic & Nuclear)The solubility of 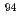 Nb in calcium alkaline solutions is one of the important parameters in safety assessment of intermediate-depth disposal which are assumed to use cementitious materials. Nb solubility and solubility-limiting solid phases of Nb in these systems remain unclear. The oversaturation solubility experiments were performed systematically in the 0.001-0.1 M CaCl
Nb in calcium alkaline solutions is one of the important parameters in safety assessment of intermediate-depth disposal which are assumed to use cementitious materials. Nb solubility and solubility-limiting solid phases of Nb in these systems remain unclear. The oversaturation solubility experiments were performed systematically in the 0.001-0.1 M CaCl solutions under alkali conditions, and the characterization of precipitated solid phase controlling Nb solubility was conducted. The negative dependence of Nb solubilities on pH and Ca concentration was observed in solubility experiments, the molar ratio of Nb to Ca of precipitated solid phase was 0.66. The pH and Ca dependence of Nb solubilities was reproduced by the reaction with Nb aqueous species Nb(OH)
solutions under alkali conditions, and the characterization of precipitated solid phase controlling Nb solubility was conducted. The negative dependence of Nb solubilities on pH and Ca concentration was observed in solubility experiments, the molar ratio of Nb to Ca of precipitated solid phase was 0.66. The pH and Ca dependence of Nb solubilities was reproduced by the reaction with Nb aqueous species Nb(OH)
 and Ca-Nb oxide with the molar ratio of Nb to Ca 0.66, e.g., Ca
and Ca-Nb oxide with the molar ratio of Nb to Ca 0.66, e.g., Ca Nb
Nb O
O (am).
(am).
Ikeuchi, Hirotomo; Koyama, Shinichi; Osaka, Masahiko; Takano, Masahide; Nakamura, Satoshi; Onozawa, Atsushi; Sasaki, Shinji; Onishi, Takashi; Maeda, Koji; Kirishima, Akira*; et al.
JAEA-Technology 2022-021, 224 Pages, 2022/10
A set of technology, including acid dissolving, has to be established for the analysis of content of elements/nuclides in the fuel debris samples. In this project, a blind test was performed for the purpose of clarifying the current level of analytical accuracy and establishing the alternative methods in case that the insoluble residue remains. Overall composition of the simulated fuel debris (homogenized powder having a specific composition) were quantitatively determined in the four analytical institutions in Japan by using their own dissolving and analytical techniques. The merit and drawback for each technique were then evaluated, based on which a tentative flow of the analyses of fuel debris was constructed.
 O
O solution
solutionKumagai, Yuta; Kusaka, Ryoji; Nakada, Masami; Watanabe, Masayuki; Akiyama, Daisuke*; Kirishima, Akira*; Sato, Nobuaki*; Sasaki, Takayuki*
Journal of Nuclear Science and Technology, 59(8), p.961 - 971, 2022/08
Times Cited Count:2 Percentile:53.91(Nuclear Science & Technology)We investigated potential degradation of fuel debris caused by H O
O , which is the oxidant of major impact from water radiolysis. We performed leaching experiments on different kinds of simulated debris comprising U, Fe, Cr, Ni, and Zr in an aqueous H
, which is the oxidant of major impact from water radiolysis. We performed leaching experiments on different kinds of simulated debris comprising U, Fe, Cr, Ni, and Zr in an aqueous H O
O solution. Chemical analysis of the leaching solution showed that U dissolution was induced by H
solution. Chemical analysis of the leaching solution showed that U dissolution was induced by H O
O . Raman analysis after the leaching revealed that uranyl peroxides were formed on the surface of the simulated debris. These results demonstrate that uranyl peroxides are possible alteration products of fuel debris from H
. Raman analysis after the leaching revealed that uranyl peroxides were formed on the surface of the simulated debris. These results demonstrate that uranyl peroxides are possible alteration products of fuel debris from H O
O reaction. However, the sample in which the main uranium-containing phase was a U-Zr oxide solid solution showed much less uranium dissolution and no Raman signal of uranyl peroxides. Comparison of these results indicates that formation of an oxide solid solution of Zr with UO
reaction. However, the sample in which the main uranium-containing phase was a U-Zr oxide solid solution showed much less uranium dissolution and no Raman signal of uranyl peroxides. Comparison of these results indicates that formation of an oxide solid solution of Zr with UO improves the stability of fuel debris against H
improves the stability of fuel debris against H O
O reaction.
reaction.
Kitagaki, Toru; Yoshida, Kenta*; Liu, P.*; Shobu, Takahisa
npj Materials Degradation (Internet), 6(1), p.13_1 - 13_8, 2022/02
Times Cited Count:1 Percentile:14.66(Materials Science, Multidisciplinary)Kitagaki, Toru
Journal of Nuclear Materials, 557, p.153254_1 - 153254_8, 2021/12
Times Cited Count:1 Percentile:16.35(Materials Science, Multidisciplinary)Kobayashi, Taishi*; Nakajima, Shogo*; Motokawa, Ryuhei; Matsumura, Daiju; Saito, Takumi*; Sasaki, Takayuki*
Langmuir, 35(24), p.7995 - 8006, 2019/06
Times Cited Count:5 Percentile:22.5(Chemistry, Multidisciplinary) O
O concentration on oxidative dissolution of U
concentration on oxidative dissolution of U O
OKumagai, Yuta
Hoshasen Kagaku (Internet), (107), p.77 - 78, 2019/04
Reaction of hydrogen peroxide (H O
O ) with uranium dioxide (UO
) with uranium dioxide (UO ) oxidizes U(IV) to water-soluble U(VI). In the concept of direct geological disposal of spent nuclear fuel, this reaction is expected to induce dissolution of UO
) oxidizes U(IV) to water-soluble U(VI). In the concept of direct geological disposal of spent nuclear fuel, this reaction is expected to induce dissolution of UO matrix of the spent fuel. This study investigate effect of H
matrix of the spent fuel. This study investigate effect of H O
O concentration on the kinetics and the yield of U(VI) dissolution of this reaction. A series of experiments of the reaction of H
concentration on the kinetics and the yield of U(VI) dissolution of this reaction. A series of experiments of the reaction of H O
O with UO
with UO powder dispersed in water has been carried out. The experimental results reveal that increase in the H
powder dispersed in water has been carried out. The experimental results reveal that increase in the H O
O concentration slows down the reaction and decreases the yield of U(VI) dissolution. This observation suggests that a reaction intermediate is generated in the course of the H
concentration slows down the reaction and decreases the yield of U(VI) dissolution. This observation suggests that a reaction intermediate is generated in the course of the H O
O reaction on the surface of UO
reaction on the surface of UO .
.
 induced by H
induced by H O
O and
and  -irradiation
-irradiationKumagai, Yuta; Fidalgo, A. B.*; Jonsson, M.*
Journal of Physical Chemistry C, 123(15), p.9919 - 9925, 2019/04
Times Cited Count:19 Percentile:62.54(Chemistry, Physical)Radiation-induced oxidative dissolution of uranium dioxide (UO ) is one of the most important chemical processes of U driven by redox reactions. We have examined the effect of UO
) is one of the most important chemical processes of U driven by redox reactions. We have examined the effect of UO stoichiometry on the oxidative dissolution of UO
stoichiometry on the oxidative dissolution of UO induced by hydrogen peroxide (H
induced by hydrogen peroxide (H O
O ) and
) and  -ray irradiation. By comparing the reaction kinetics of H
-ray irradiation. By comparing the reaction kinetics of H O
O between stoichiometric UO
between stoichiometric UO and hyper-stoichiometric UO
and hyper-stoichiometric UO , we observed a significant difference in reaction speed and U dissolution kinetics. The stoichiometric UO
, we observed a significant difference in reaction speed and U dissolution kinetics. The stoichiometric UO reacted with H
reacted with H O
O much faster than the hyper-stoichiometric UO
much faster than the hyper-stoichiometric UO . The U dissolution from UO
. The U dissolution from UO was initially much lower than that from UO
was initially much lower than that from UO , but gradually increased as the oxidation by H
, but gradually increased as the oxidation by H O
O proceeded. The
proceeded. The  -ray irradiation induced the U dissolution that is analogous to the kinetics by the exposure to a low concentration (0.2 mM) of H
-ray irradiation induced the U dissolution that is analogous to the kinetics by the exposure to a low concentration (0.2 mM) of H O
O . The exposure to higher H
. The exposure to higher H O
O concentrations caused lower U dissolution and resulted in deviation from the U dissolution behavior by
concentrations caused lower U dissolution and resulted in deviation from the U dissolution behavior by  -ray irradiation.
-ray irradiation.
Hayashi, Hirokazu; Chiba, Rikiya*
Progress in Nuclear Science and Technology (Internet), 5, p.196 - 199, 2018/11
Uranium-free nitride fuel has been chosen as the first candidate for transmutation of long-lived minor actinides (MA: Np, Am, Cm) using sub-critical accelerator-driven system (ADS) under the double strata fuel cycle concept by Japan Atomic Energy Agency (JAEA). Dissolution behavior of ZrN-based nitrides in nitric acid is examined using lanthanides as surrogate materials of TRU elements. Chemical analysis of the ZrN-based lanthanide nitrides dissolved in nitric acid is also carried out.
Tanno, Takashi; Takeuchi, Masayuki; Otsuka, Satoshi; Kaito, Takeji
Journal of Nuclear Materials, 494, p.219 - 226, 2017/10
Times Cited Count:17 Percentile:85.35(Materials Science, Multidisciplinary)Oxide dispersion strengthened (ODS) steel cladding tubes have been developed for fast reactors. 9 chromium ODS and 11Cr-ODS tempered martensitic steels are prioritized for the candidate material in research being carried out at JAEA. In this work, fundamental immersion tests and electro-chemical tests of 9 to 12Cr-ODS steels were systematically conducted in various nitric acid solutions at 95 C. The corrosion rate exponentially decreased with effective solute chromium concentration (Cr
C. The corrosion rate exponentially decreased with effective solute chromium concentration (Cr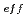 ) and nitric acid concentration. Addition of oxidizing ions also suppressed the corrosion rate. According to polarization curves and surface observations in this work, the combination of low Cr
) and nitric acid concentration. Addition of oxidizing ions also suppressed the corrosion rate. According to polarization curves and surface observations in this work, the combination of low Cr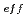 and dilute nitric acid could not prevent the active dissolution at the beginning of immersion, and the corrosion rate was high. In comparison, higher Cr
and dilute nitric acid could not prevent the active dissolution at the beginning of immersion, and the corrosion rate was high. In comparison, higher Cr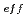 , concentrated nitric acid and addition of oxidizing ions helped to prevent the active dissolution, and suppressed the corrosion rate.
, concentrated nitric acid and addition of oxidizing ions helped to prevent the active dissolution, and suppressed the corrosion rate.
Oe, Toshiaki*; Wakasugi, Keiichiro
Genshiryoku Bakkuendo Kenkyu (CD-ROM), 24(1), p.27 - 32, 2017/06
The report estimates the life-time of the waste glass dissolution in the geologic disposal environment. The overall safety report on the geologic disposal in Japan showed very short life-time of approximately 70,000 years under pessimistic assumptions ignoring the temperature decrease due to radioactive decay and dissolution rate reduction due to surface shrinkage. These factors are physically established phenomena and may not be excluded. The dissolution models including these factors of temperature and surface area decreases are discussed and used for re-evaluation. Three fracture models are presented for evaluating the surface area decreases; a single plate, monotonic spheres, spheres having power-law distribution. All models have the same initial volume as the waste glass block for mass conservation and the total surface areas are 10 times higher than the initial pristine block because of the fracture development during production. The results indicate the retention time of 50% of initial mass exceed 100,000 years even by different fracture models and the dissolution life-times are expected for 260,000 700,000 years depending on models. These results imply more strong isolation capability of the waste glass than that estimated in the overall safety report.
700,000 years depending on models. These results imply more strong isolation capability of the waste glass than that estimated in the overall safety report.
Shimada, Taro; Nishimura, Yuki; Takeda, Seiji
MRS Advances (Internet), 2(12), p.687 - 692, 2017/01
A disposal measure for fuel debris generated at the accident in the Fukushima Daiichi Nuclear Power Station has been studied so far. However, physical and chemical properties of the fuel debris have not yet investigated in reactor containment vessels. In order to investigate the safety function of barriers required for disposal of fuel debris, sensitivity analyses for radionuclide migration were carried out, considering with uncertainty of the properties. As a result, it is indicated that it was important for evaluation of fuel debris disposal to obtain the physical and chemical properties of  C and
C and  I during release to groundwater, in addition to
I during release to groundwater, in addition to  U.
U.
Kitamura, Akira; Chikazawa, Takahiro*; Akahori, Kuniaki*; Tachi, Yukio
Genshiryoku Bakkuendo Kenkyu (CD-ROM), 23(1), p.55 - 72, 2016/06
The Japanese geological disposal program has started researching disposal of spent nuclear fuel (SF) in deep geological strata (hereafter "direct disposal of SF") as an alternative management option other reprocessing followed by vitrification and geological disposal of high-level radioactive waste. We conducted literature survey of dissolution rate of SF matrix and constructing materials (e.g. zircaloy cladding and control rods) selected in safety assessment reports for direct disposal of SF in Europe and United States. We also investigated basis of release rate determination and assignment of uncertainties in the safety assessment reports. Furthermore, we summarized major conclusions proposed by some European projects governed by European Commission. It was found that determined release rates are fairly similar to each other due to use of similar literature data in all countries of interest. It was also found that the determined release rates were including conservativeness because it was difficult to assign uncertainties quantitatively. It is expected that these findings are useful as fundamental information for determination of the release rates for the safety assessment of Japanese SF disposal system.
Konda, Miki; Asai, Shiho; Hanzawa, Yukiko; Magara, Masaaki
JAEA-Technology 2015-054, 22 Pages, 2016/03
Isotope dilution mass spectrometry (IDMS) with ICP-MS is reliable method for determination of Zr-93, which is one of the long-lived fission products found in spent nuclear fuel and high-level radioactive wastes. In order to use an isotope standard solution of zirconium as the spike for IDMS, dissolving a commercially available solid isotope standard is indispensable. Prior to the dissolution of the Zr-91 isotope standard, solubility of metal zirconium in a mixture of HNO and HF was evaluated using zirconium metal chips. Then, 2 mg of the Zr-91 isotope standard was dissolved with 0.2 mL of 1 M HNO
and HF was evaluated using zirconium metal chips. Then, 2 mg of the Zr-91 isotope standard was dissolved with 0.2 mL of 1 M HNO -3 v/v% HF mixed solution, followed by adjusting the concentration of Zr-91 to approximately 1,000
-3 v/v% HF mixed solution, followed by adjusting the concentration of Zr-91 to approximately 1,000  g/g. IDMS, in which a natural isotopic abundance standard solution of zirconium was used as the spike, was employed for the determination of the concentration of Zr-91 in the prepared Zr-91 isotope standard solution. The concentration of Zr-91 in the prepared Zr-91 isotope standard solution was (9.6
g/g. IDMS, in which a natural isotopic abundance standard solution of zirconium was used as the spike, was employed for the determination of the concentration of Zr-91 in the prepared Zr-91 isotope standard solution. The concentration of Zr-91 in the prepared Zr-91 isotope standard solution was (9.6 1.0)
1.0)  10
10
 g/g, which is in good agreement with the predicted concentration. This indicates that the Zr-91 metal isotope standard was completely dissolved with sufficient chemical stability. Additionally, no impurities were detected in the prepared Zr-91 isotope standard solution. These positive results denote that the Zr-91 isotope standard solution with the preferable quality for IDMS of Zr-93 can be obtained by the proposed dissolution procedures.
g/g, which is in good agreement with the predicted concentration. This indicates that the Zr-91 metal isotope standard was completely dissolved with sufficient chemical stability. Additionally, no impurities were detected in the prepared Zr-91 isotope standard solution. These positive results denote that the Zr-91 isotope standard solution with the preferable quality for IDMS of Zr-93 can be obtained by the proposed dissolution procedures.
 C based on two binary non-ideal solid solutions
C based on two binary non-ideal solid solutionsWalker, C.; Suto, Shunkichi; Oda, Chie; Mihara, Morihiro; Honda, Akira
Cement and Concrete Research, 79, p.1 - 30, 2016/01
Times Cited Count:69 Percentile:90.65(Construction & Building Technology)Modeling the solubility behavior of calcium silicate hydrate (C-S-H) gel is important to make quantitative predictions of the degradation of hydrated ordinary Portland cement (OPC) based materials. Experimental C-S-H gel solubility data have been compiled from the literature, critically evaluated and supplemented with new data from the current study for molar Ca/Si ratios = 0.2-0.83. All these data have been used to derive a discrete solid phase (DSP) type C-S-H gel solubility model based on two binary non-ideal solid solutions in aqueous solution(SSAS). Features of the DSP type C-S-H gel solubility model include satisfactory predictions of pH values and Ca and Si concentrations for all molar Ca/Si ratios = 2.7  0 in the C-S-H system, portlandite (CH) for Ca/Si ratios
0 in the C-S-H system, portlandite (CH) for Ca/Si ratios  1.65, congruent dissolution at Ca/Si ratios = 0.85, and amorphous silica (SiO
1.65, congruent dissolution at Ca/Si ratios = 0.85, and amorphous silica (SiO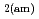 ) for Ca/Si ratios
) for Ca/Si ratios  0.55 as identified in the current study by IR spectroscopy.
0.55 as identified in the current study by IR spectroscopy.
Yamagishi, Isao; Odakura, Makoto; Ichige, Yoshiaki; Kuroha, Mitsuhiko; Takano, Masahide; Akabori, Mitsuo; Yoshioka, Masahiro*
Proceedings of 21st International Conference & Exhibition; Nuclear Fuel Cycle for a Low-Carbon Future (GLOBAL 2015) (USB Flash Drive), p.1113 - 1119, 2015/09
The characteristics of insoluble residues in fine suspension at the Rokkasho Reprocessing Plant were analyzed. The insoluble residues were washed with oxalic acid solution to dissolve zirconium molybdate residues. XRD profiles of unwashed residues showed the presence of a noble metal alloy, zirconium molybdate, and zirconia, but zirconium molybdate was not found after washing. More than 50% of the Sb-125 and Pu in thee residues was washed out as well. The noble metal alloy composed of Mo, Tc, Ru, Rh, and Pd occupied more than 90% of the total weight of 12 elements (Ca, Cr, Fe, Ni, Zr, Mo, Tc, Ru, Rh, Pd, Te, and U) found in the residues. In consideration of the chemical forms of 12 elements, the alloy-to-residue weight ratio was evaluated to be 64% and 78% with and without 18% of an unknown component, respectively.
Committee of Handbook on Process and Chemistry of Nuclear Fuel Reprocessing
JAEA-Review 2015-002, 726 Pages, 2015/03
The fundamental data on spent nuclear fuel reprocessing and related chemistry was collected and summarized as a new edition of "Handbook on Process and Chemistry of Nuclear Fuel Reprocessing". The purpose of this handbook is contribution to development of the fuel reprocessing and fuel cycle technology for uranium fuel and mixed oxide fuel utilization. Contents in this book was discussed and reviewed by specialists of science and technology on fuel reprocessing in Japan.
Oe, Kazuhiro*; Attallah, M. F.*; Asai, Masato; Goto, Naoya*; Gupta, N. S.*; Haba, Hiromitsu*; Huang, M.*; Kanaya, Jumpei*; Kaneya, Yusuke*; Kasamatsu, Yoshitaka*; et al.
Journal of Radioanalytical and Nuclear Chemistry, 303(2), p.1317 - 1320, 2015/02
Times Cited Count:10 Percentile:64.63(Chemistry, Analytical)A new technique for continuous dissolution of nuclear reaction products transported by a gas-jet system was developed for superheavy element (SHE) chemistry. In this technique, a hydrophobic membrane is utilized to separate an aqueous phase from the gas phase. With this technique, the dissolution efficiencies of short-lived radionuclides of 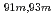 Mo and
Mo and 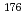 W were measured. Yields of more than 80% were observed for short-lived radionuclides at aqueous-phase flow rates of 0.1-0.4 mL/s. The gas flow-rate had no influence on the dissolution efficiency within the studied flow range of 1.0-2.0 L/min. These results show that this technique is applicable for on-line chemical studies of SHEs in the liquid phase.
W were measured. Yields of more than 80% were observed for short-lived radionuclides at aqueous-phase flow rates of 0.1-0.4 mL/s. The gas flow-rate had no influence on the dissolution efficiency within the studied flow range of 1.0-2.0 L/min. These results show that this technique is applicable for on-line chemical studies of SHEs in the liquid phase.
Maeda, Toshikatsu; Bamba, Tsunetaka*; Mizuno, Tsuyoshi*; Terakado, Shogo; Kitagawa, Isamu; Numata, Masami
Haikibutsu Gakkai Rombunshi, 17(4), p.271 - 281, 2006/07
no abstracts in English
Maeda, Toshikatsu; Bamba, Tsunetaka*; Hotta, Katsutoshi*; Mizuno, Tsuyoshi*; Ozawa, Tatsuya
Nihon Genshiryoku Gakkai Wabun Rombunshi, 4(4), p.242 - 247, 2005/12
no abstracts in English